What Happens When Strong Acid Reacts With Strong Base . titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Ha + boh → ba + h 2 o + heat. Strong acid with strong base: when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): As one can see, most of the strong bases. mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. A number of common strong acids, including. when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. This is called a neutralization reaction and looks like this:
from www.flinnsci.ca
A number of common strong acids, including. Ha + boh → ba + h 2 o + heat. when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. As one can see, most of the strong bases. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Strong acid with strong base: This is called a neutralization reaction and looks like this:
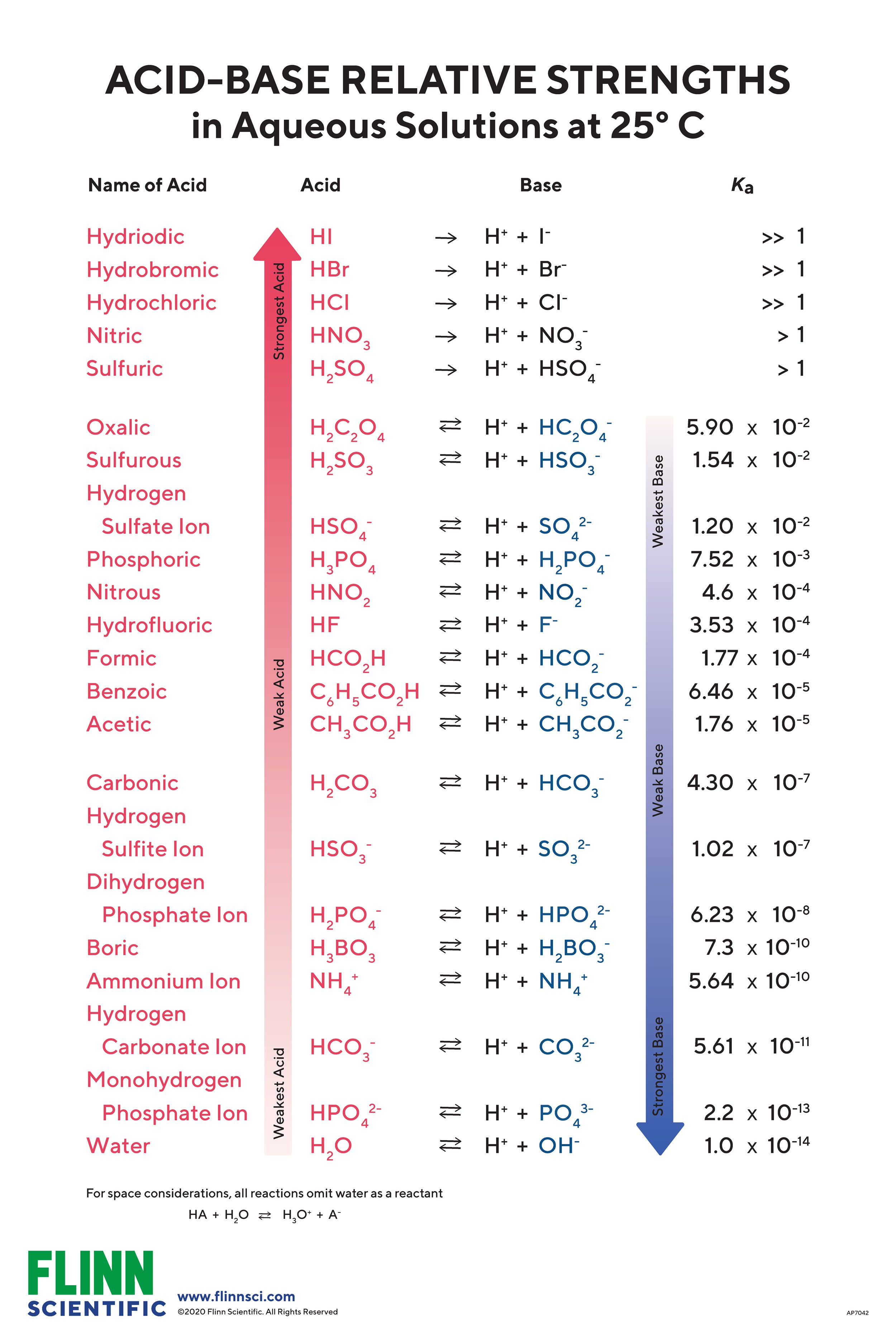
AcidBase Strength Charts for Chemistry
What Happens When Strong Acid Reacts With Strong Base Ha + boh → ba + h 2 o + heat. As one can see, most of the strong bases. when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. This is called a neutralization reaction and looks like this: A number of common strong acids, including. Ha + boh → ba + h 2 o + heat. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Strong acid with strong base: mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution.
From www.chegg.com
Solved REACTION OF ACIDS AND BASES 1. The reaction between a What Happens When Strong Acid Reacts With Strong Base Strong acid with strong base: titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This is called a neutralization reaction and looks like this: mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph. What Happens When Strong Acid Reacts With Strong Base.
From mavink.com
Acid Base Titration Equation What Happens When Strong Acid Reacts With Strong Base A number of common strong acids, including. This is called a neutralization reaction and looks like this: when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. Strong acid with strong base: An example would be the reaction between the strong acid hcl (hydrochloric acid). What Happens When Strong Acid Reacts With Strong Base.
From www.slideserve.com
PPT Strong Acids and Bases PowerPoint Presentation, free download What Happens When Strong Acid Reacts With Strong Base This is called a neutralization reaction and looks like this: Ha + boh → ba + h 2 o + heat. mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh. What Happens When Strong Acid Reacts With Strong Base.
From www.youtube.com
Weak basestrong acid reactions Acids and bases AP Chemistry Khan What Happens When Strong Acid Reacts With Strong Base Ha + boh → ba + h 2 o + heat. This is called a neutralization reaction and looks like this: As one can see, most of the strong bases. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): mixing equal amounts of a strong acid with a. What Happens When Strong Acid Reacts With Strong Base.
From www.slideserve.com
PPT Chapter 11 Alcohols and Ethers PowerPoint Presentation ID300959 What Happens When Strong Acid Reacts With Strong Base Ha + boh → ba + h 2 o + heat. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized. What Happens When Strong Acid Reacts With Strong Base.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube What Happens When Strong Acid Reacts With Strong Base when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. Strong acid with strong base: mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong. What Happens When Strong Acid Reacts With Strong Base.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs What Happens When Strong Acid Reacts With Strong Base when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This is called a neutralization reaction and looks. What Happens When Strong Acid Reacts With Strong Base.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive What Happens When Strong Acid Reacts With Strong Base This is called a neutralization reaction and looks like this: As one can see, most of the strong bases. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. mixing equal amounts of a strong acid with a strong base also produces. What Happens When Strong Acid Reacts With Strong Base.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Happens When Strong Acid Reacts With Strong Base when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. Ha + boh → ba + h 2 o + heat. This is called a neutralization reaction and looks like this: titration of a strong acid with a strong base is the simplest of. What Happens When Strong Acid Reacts With Strong Base.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry What Happens When Strong Acid Reacts With Strong Base An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): Ha + boh → ba + h 2 o + heat. A number of common strong acids, including. mixing equal amounts of a strong acid with a strong base also produces a neutral ph (ph = 7) solution. As. What Happens When Strong Acid Reacts With Strong Base.
From saylordotorg.github.io
AcidBase Titrations What Happens When Strong Acid Reacts With Strong Base Strong acid with strong base: when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Ha + boh. What Happens When Strong Acid Reacts With Strong Base.
From stock.adobe.com
Strong acid and strong base reaction. Strengths of acids and bases What Happens When Strong Acid Reacts With Strong Base An example would be the reaction between the strong acid hcl (hydrochloric acid) with the strong base naoh (sodium hydroxide): titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. A number of common strong acids, including. Ha + boh → ba +. What Happens When Strong Acid Reacts With Strong Base.
From mavink.com
Acid Base Reaction Graph What Happens When Strong Acid Reacts With Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. As one can see, most of the strong bases. This is called a neutralization reaction and looks like this: mixing equal amounts of a strong acid with a strong base also produces. What Happens When Strong Acid Reacts With Strong Base.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry What Happens When Strong Acid Reacts With Strong Base when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. This is called a neutralization reaction and looks like this: titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. Strong acid with strong base:. What Happens When Strong Acid Reacts With Strong Base.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube What Happens When Strong Acid Reacts With Strong Base when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized red form, in. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. A number of common strong acids, including. Strong. What Happens When Strong Acid Reacts With Strong Base.
From www.youtube.com
How To Memorize The Strong Acids and Strong Bases YouTube What Happens When Strong Acid Reacts With Strong Base titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. This is called a neutralization reaction and looks like this: Ha + boh → ba + h 2 o + heat. mixing equal amounts of a strong acid with a strong base. What Happens When Strong Acid Reacts With Strong Base.
From www.slideserve.com
PPT Acids and bases, salts and solutions PowerPoint Presentation What Happens When Strong Acid Reacts With Strong Base when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong acid and strong. As one can see, most of the strong bases. A number of common strong acids,. What Happens When Strong Acid Reacts With Strong Base.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo What Happens When Strong Acid Reacts With Strong Base when a strong acid and a strong base are combined in the proper amounts—when \([\ce{h^{+}}]\) equals \([\ce{oh^{. Ha + boh → ba + h 2 o + heat. As one can see, most of the strong bases. when we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the unionized. What Happens When Strong Acid Reacts With Strong Base.